|
|
|
|
|
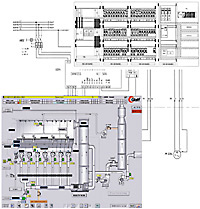 |
Automation solutions for the whole
process.
Customized systems.
Based
on globally established standards.
Integration.
In networks.
In
higher-level systems.
Modular design.
Convenient operating and visualization
facilities.
Compliant with 21 CFR Part 11.
|
| |
|
|
Automation solutions for the whole process
Glatt provides modern, economic and future-orientated automation solutions
for the control of complete process lines right up to the control of the whole
manufacturing process incl. logistics and auxiliary processes.
|
|
|
Customized systems
In order to fulfill customer-specific requirements to the greatest possible
extent, we develop and implement our own control systems for machines and
processes. Globally established software and hardware form the basis of these.
Glatt provides applications based on Siemens PCS 7, Intellution, Citect,
Wonderware or WinCC.
|
|
|
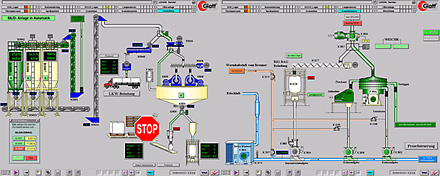
Process visualization, transportation of solid material |
|
|
The advantages for you:
Individual control system and world-wide local support.
Your maintenance personnel do not need special programming knowledge to
diagnose faults.
This guarantees high availability for your system.
|
|
|
Integration into networks and higher-level systems
Interfaces for integration into networks and higher-level systems such as an
MES (Manufacturing Execution System) or SAP. Use of
industrial standards such as Ethernet, TCP/IP.
Control of processes in
different production locations.
Remote diagnostics and remote maintenance
facilities.
|
|
|

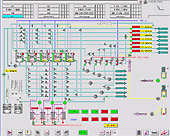
Control room |
| |
Modular design
Pre-parameterised modules allow maximum flexibility when engineering and
scaling all machine-specific components of the control system.
|
| |
Convenient operating and visualization facilities
Process visualization for all control stations.
Comprehensive information
on current operating conditions.
Recording, processing and archiving of all
relevant process data.
Plausible process guidance for error-free
control.
Monitoring and reporting of alarm, fault and operator messages.
|
| |
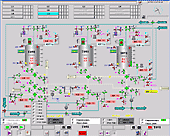
  Process visualization, dairy line
Process visualization, dairy line |
 |
|
| |
Compliant with 21 CFR Part 11.
Measurements are recorded, processed and stored electronically. Connection to
databases, electronic records (ER), electronic signatures (ES) or audit trail -
Glatt process control systems fulfill the requirements of the FDA Directive 21
CFR Part 11. And of course, if you wish, we will be pleased to provide you with
comprehensive documentation on our control systems for Validation, Qualification and Calibration - right up to GAMP 4.
|